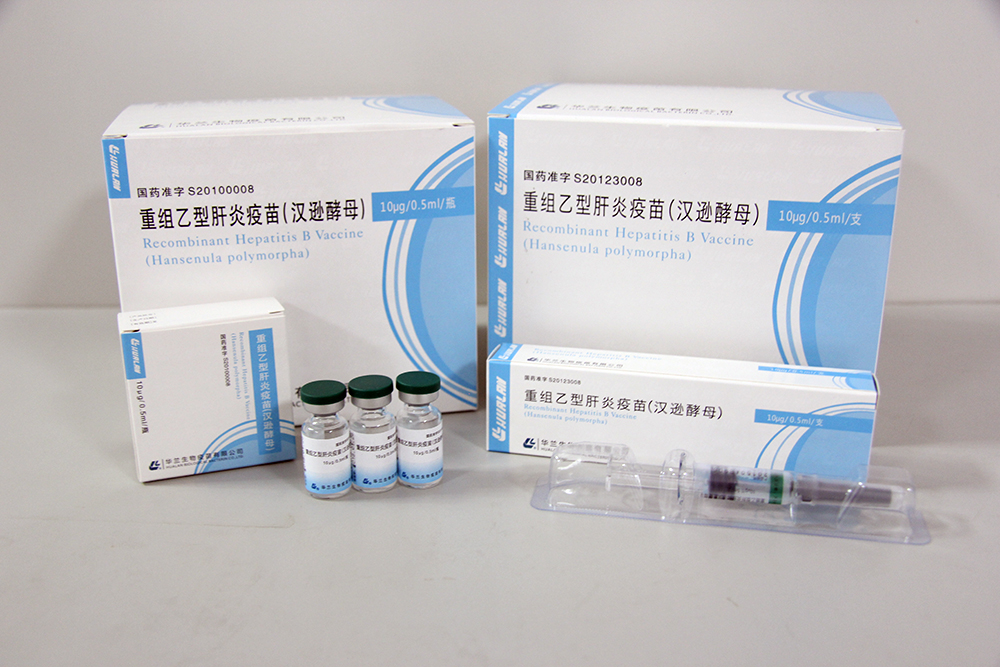
10μg /0.5mL/syringe
| 【GENERIC NAME】 |
| Recombinant Hepatitis B Vaccine (Hansenula Polymorpha) |
| 【VACCINATION SUBJECTS】 |
| The subject aged 5 years and above susceptible to hepatitis B virus, particularly for the health care personnel or the laboratory workers handling human blood. |
- 10μg /0.5mL/syringe
- 10μg /0.5mL/vial
[Drug Name]
Generic Name: Recombinant Hepatitis B Vaccine (Hansenula polymorpha)
English Name: Recombinant Hepatitis B Vaccine (Hansenula polymorpha)
Chinese Pinyin: Chongzu Yixing Ganyan Yimiao (Hanxun Jiaomu)
[Constituents and Characters]
The product is prepared by purifying hepatitis B virus surface antigen (HBsAg) expressed by recombinant Hansenula polymorpha and adding aluminum adjuvant. It is a milky white suspension, which can be layered due to precipitation and is easy to shake.
Excipients: Al(OH)3, Na2HPO4, NaH2PO4 and NaCl.
[Vaccination Subjects]
Subjects below 16 years old who is susceptible to hepatitis B virus, particularly for the following people:
(1) Newborns, especially those whose mothers are HBsAg-positive or/and HBeAg-positive;
(2) Health care personnel or the laboratory workers contacting human blood.
[Function and Use]
The product can induce immunity against hepatitis B virus in recipients following immunization. It is used to prevent hepatitis B.
[Specifications]
0.5 ml per container.0.5 ml per single human dose containing 10μg of HBsAg.
[Administration and Dosages]
(1) Intramuscularly inject the vaccine in the region of the deltoid muscle of the upper arm.
(2) Three injections are required for a complete immunization course, which is injected at the time points of the 0th, 1st and 6th month. The first dose of vaccine shall be injected for each newborn within 24 hours after birth, and one dose for each injection.
[Adverse Reactions]
Common adverse reactions:
Pain and haphalgesia at the injection site may appear within 24 hours after vaccination. Normally it will disappear in two or three days.
Rare adverse reactions:
(1) Transient fever may appear within 72 hours after vaccination. Normally it lasts 1~2 days and will vanish without treatment.
(2) Mild and moderate pain and swelling may appear at injection site and will be relieved spontaneously within 1~2 days without treatment.
Very rare adverse reactions:
(1) Indurations at injection site may appear, and will be absorbed within 1~2 months.
(2) Local aseptic suppuration: The pus should be pumped out repeatedly with syringes. If serious, debridement should be given to remove necrotic tissues. It will last a longer time, but will eventually heal.
(3) Anaphylaxis: Allergic rash and Arthur reaction. Arthur reaction generally occurs within 10 days after vaccination, with reddish swelling at local site lasting a long time. Sterols may be used for systemic and/or local treatment.
(4) Anaphylactic shock: generally occurs within 1 hour after vaccination. Adrenaline should be given timely for treatment.
[Contraindications]
(1) People with known hypersensitivity reactions to any components of the vaccine, including yeast and excipients.
(2) People who have acute illness, severe chronic diseases, acute exacerbation of chronic disease and fever.
(3) Pregnant women.
(4) People with uncontrolled epilepsy or other progressive neurologic disorders.
[Precautions]
(1) The vaccine should be used with caution in the following cases: Family and the individual have a history of convulsions, chronic disease, a history of epilepsy, allergy.
(2) Shake the container well before use. Do not use the vaccine if any foreign matter or the clump not dispersed on shaking, leakage of vial, illegible label are found.
(3) Administer immediately once opened.
(4) Adrenaline should be available for first aid in case of severe anaphylactic reactions. The vaccinees shall take a rest for at least 30 minutes on site following immunization.
(5) If abnormal conditions such as high fever or convulsion occur after the first injection, the second injection should not be given.
(6) Do not freeze.
[Storage] Store and ship at 2-8°C, protected from light.
[Packaging]10μg/0.5ml/vial, 3 vials per carton, packaged by colorless glass tubing vial and brominated butyl rubber stopper.
[Shelf Life]36 months.
[Product Standard] YBS00522011 and Chinese Pharmacopoeia (2015) Volume III
[Product License No.]GYZZ S20100008
[Manufacturer]
Hualan Biological Bacterin Inc.
Address: Jia No.1-1, Hualan Ave., Xinxiang, Henan, China
Tel: 86-373-3559991
Fax: 86-373-3559911
- UP:NONE;
- NEXT:10μg /0.5mL/vial